FRANKLIN MARBLE
Significant zinc ore bodies in the Franklin Marble of
Mesoproterozoic age have been mined in northern New Jersey, USA for many
decades. The sample shown below is a zinc ore from Sterling Hill, New
Jersey. The two zinc minerals here are black franklinite
((Zn,Fe)Fe2O4 - zinc iron oxide) & red zincite
(ZnO - zinc oxide).
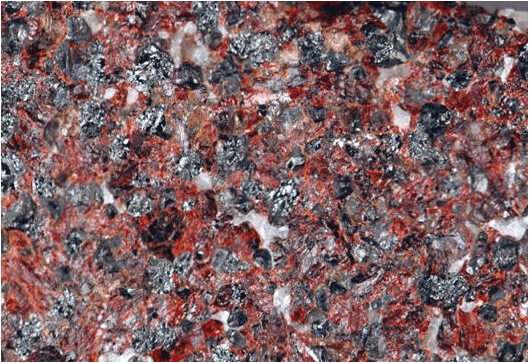
Zinc ore
(= zincite-franklinite-calcite rock) (above & below; above: ~6.2 cm
across; below: ~2.1 cm across)

The New Jersey zincite-franklinite bodies are
traditionally considered as skarn deposits - the result of contact metamorphism
of Cambro-Ordovician limestones by igneous intrusions. Elsewhere, igneous
intrusion of limestones does result in the formation of odd mineral suites by
contact metamorphism.
However, these zinc ores do not appear to be skarn
deposits. Their exact origin is still debated in the literature, but
published research suggests that the zinc ore bodies were originally Zn-rich
metalliferous sediments deposited in the margin of a marine basin. The
marine basin was subsequently metamorphosed by subduction during the Grenville
Orogeny (1.03-1.08 billion years ago) and became enclosed in marble host rocks
by inverse diapirism.
In addition to their economic geologic significance, the
rocks and minerals from the Franklin and Sterling Hill zinc orebodies of New
Jersey are famous for their gorgeous fluorescent colors under ultraviolet (UV)
light.
The rocks shown below are from the Franklin zinc
orebody in the Mesoproterozoic-aged Franklin Marble at Franklin, New
Jersey. They contain three minerals. Under white light, the
dominant mineral is whitish-grayish in color - that's calcite (CaCO3
- calcium carbonate). Calcite is what makes marble marble.
Under UV light, manganiferous calcite will fluoresce an intense orangish color
(see below).
The black mineral is franklinite ((Zn,Fe,Mn)(Fe,Mn)2O4)
- zinc iron manganese oxide), a dominant zinc ore mineral in New Jersey.
It does not fluoresce under black light.
The light brown to peachy-colored mineral is willemite
(Zn2SiO4 - zinc silicate). Willemite varies
considerably in color under white light, but will always have an intense
greenish fluorescence under UV light (see below).

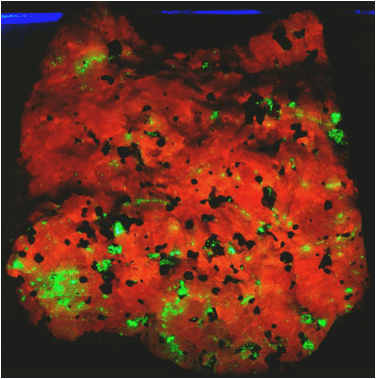
Zinciferous marble (above & below) (5.5 cm across) from the
Mesoproterozoic-aged Franklin Marble of Franklin, New Jersey, USA under white
light (above) and ultraviolet black light (below).
Above:
white = calcite; light brown = willemite; black = franklinite.
Below:
orangish-red fluorescence = calcite; green fluorescence = willemite; black =
franklinite.

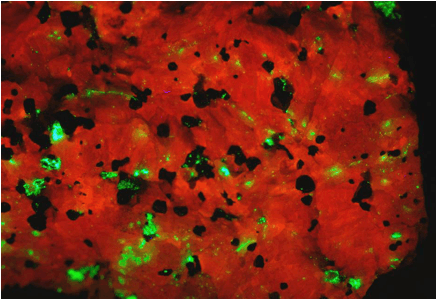
Zinciferous marble (above & below) (4.4 cm across) from the Franklin
Marble of New Jersey under white light (above) and ultraviolet black light
(below).
Above:
white = calcite; light brown = willemite; black = franklinite.
Below:
orangish-red = calcite; green = willemite; black = franklinite.
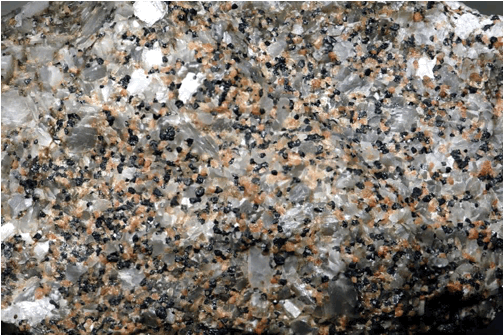
Zinciferous marble (above & below) (field of view 7.4 cm across)
from the Franklin Marble of New Jersey under white light (above) and UV black
light (below).
Above:
whitish-grayish = calcite; peachy colored = willemite; black = franklinite.
Below:
orangish-red = calcite; green = willemite; black = franklinite.
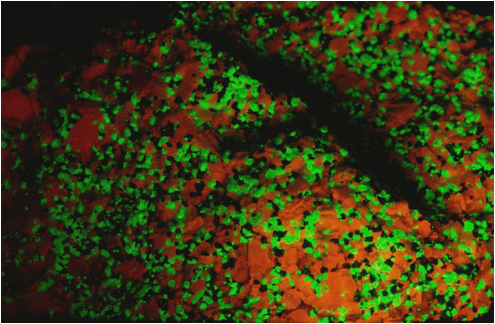
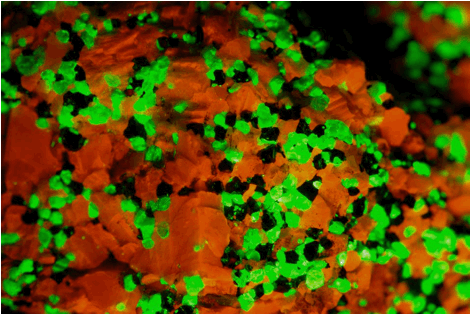
Zinciferous marble (7.4 cm across) from the Franklin Marble of New
Jersey under ultraviolet/black light.
Orangish-red = calcite; green = willemite; black =
franklinite.
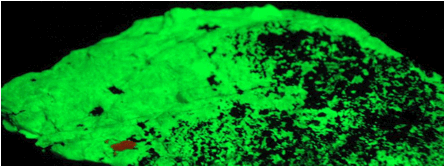
Zinc ore
under black light (UV light). Green = willemite. Black = zincite
& franklinite (not fluorescent). (OSU public display, Orton Geology
Museum, Ohio State University, Columbus, Ohio, USA)
Why do some minerals fluoresce under UV light?
When short-wavelength UV radiation, long-wavelength UV radiation, or x-rays
bombard atoms, electron excitation occurs. But the electrons do not
remain in an energetically excited state. They quickly give off energy
and resume their normal energy levels. If the electron energy release is
in the visible spectrum of light, a mineral glows, or fluoresces.